Ph of Water at Different Temperatures
There is a pH 686 and a pH 741 using a mixture of Disodium Hydrogen Phosphate and Potassium Dihydrogen Phosphate. A pH higher than 7 is basic alkaline.

Etude House Soon Jung Ph 6 5 Whip Cleanser 70ml For Sale Online Ebay Cleanser Gentle Face Scrub Safe Skincare
Such an option is generally.

. There is more dissociation at higher temperature. Recall that auto-ionisation is endothermic. This means that each 10 change in pH positive or negative is a difference of a factor of 10.
Chemistry 4th Edition Edit edition Solutions for Chapter 8 Problem 82AP. Which of the following represents the variations in the. The extent of the ionization increases with temperature.
Water consists of OH- and H3O ions whose pH will be denoted as log c H3O. Due to the logarithmic nature of pH it is quite counterintuitive that an increase in H aq actually decreases the pH. It is clearly evident from the table that the pH of water at 0oC is 747 but the same water at 100C will have a pH of 614.
2 at -35 C pH 85 112 0112 x 10-14 mol2 l-2 at 0 C pH 75 to 0991 x 10-14 mol2 l-2 at 25 C pH 70 to 9311 x 10 -14 mol 2 l -2 at 60 C pH 65 87 to 10 -12 mol 2 l. The value of Kw for water at body temperature 37 C is 21 x 10-14. This fact is clearly evident when we measure the pH of water 0 deg C we find it to be 747 but the same water at 100 deg C will have a pH of 614.
Buffer Solution pH 700 Color-coded Yellow Product 22835XX is prepared with the same composition just at different ratios to give pH 7 at 25 C. That is the neutral point on the pH scale at this. With changes in temperature the chemical equilibrium of water will also change.
When there is a change in temperature K w H concentration and therefore pH will change. The graph above shows the pH of pure water at different temperatures. 10 rows temperature conditions in a water bath for example at 20C or 25C.
At first glance this seems hardly surprising but pH of water is 7 only when K w 10-14 at 25 o C. Deduce pH of water when temperature is above 298K. At 100C the pH of pure water is 614 which is neutral on the pH scale at this higher temperature.
The higher the temperature the lower the pKw this means Kw is increasing ie. Which gives p H p O H 65 65 13 not 14. Question 35 a 80 76 72 pH 68 64 60 0 10 20 30 40 50 60 70 80 90 Temperature C In pure water some of the molecules ionize according to the equation HO H OH.
The pH of water at room temperature is 7 and when it is subjected to a high temperature of 100C the pH drops to 614 which is neutral but slightly lower than 7. Does this also mean dipping a litmus paper into 2 beakers of water of different temperature would yield different results. A student heats pure water and records the measured pH at 50C as 66.
I found the molarity of the H3O ions to be 14 x 10-7 M from taking the square root of the Kw given in the problem since Kw H3O OH-. Typical pH values for solutions at different temperatures From the table we can conclude that the effect of temperature is greatest for highly basic solutions. Water with a pH less than 65 is considered acidic.
PH is measured on a logarithmic scale. Calculate the pH of neutral water at three different temperatures taking data from Table 8-1 and use the results in conjunction with Le Chateliers principle to decide whether the autoionization of water is exothermic or endothermic. This means that neutral pH of 700 only applies at 25C.
1 10 65 65 p O H. PKw is equal to 1400 only at 25C. For water at 60 degrees Celsius K w 1 10 13 H X O H X Hence H X 1 10 65 O H X So p H log.
Although the pH of pure water is 7 drinking water and natural water exhibits a pH range because it contains dissolved minerals and gases. The aim was to examine the influence of temperatures -20 to 80 C and pH values 2 to 12 on the survival of environmental and clinical isolates of A. Bacterium Acinetobacter baumannii is an emergent pathogen associated with nosocomial infections which can be also found in natural waters.
Common Oversight with pH testing. A pH of 7 pure water is neutral. I dont know of a formula for pKw but here is a table.
The average pH for sea water is. 84K views View upvotes. A What is the molarity of H3O ions and the pH of neutral water at 37 C.
And less than 7 is acidic. Yes at 37315 K the pH value of water is actually around 614 the concentration of H aq is around 716 10 mol dm at that temperature. Yes it does if you have very good litmus paper and a sharp eye if one beaker was 273K pH 747 and another.
Toews et al. Baumannii is insufficiently investigated. Based on this information which of the following mathematical relationships gives the pOH of pure water at 50C.
Surface waters typically range from pH 65 to 85 while groundwater ranges from pH 6 to 85. For pure water pH 12 pKw. The impact of ecological factors on A.
8 rows At 100C the pH of pure water is 614. Thus a pH of 80 is 10 times more basic than 70 and 100 times more basic than 60. This water typically is corrosive and soft.
Then to find the pH of the water at body temperature. Concentrated on pH in the sub-critical regime at a temperature of 29515 K as 095 to 80 MPa which they reported as dropping from 278 to 274 as pressure. This video works through how to solve for th.
P H p O H 14. 81 In pure water some of the molecules ionize according to the equation H2OHOH. A solution with a pH of 7 at this temperature is slightly alkaline because its pH is a bit higher than the neutral value of 614.
Covered a temperature range up to 343 K as the CO 2 pressure increased from 71 to 20 MPa and reported a pH decrease from 283 to 280 as pressure increased while Parton et al. You may have learned that the pH of pure water is 7 but thats not true unless the temperature is 25 Celsius. The extent of the ionization increases with temperature.
In SM 4500-H B there is not a formulation for a buffer that would be pH 700 at 25 C.

Inactivation Of Chironomid Larva In Water With Bacillus Thuringiensis Subsp Israelensis Wastewater Treatment Bacillus Wastewater

Co2 Ph Kh Table Aquarium Plants Planted Aquarium Aquarium Fish Tank

Inline Filter Hollow Fibre Ultrafine Drinking 0 1 Micron 1 4 Fitting 6 28 10 22 Water Filter Drinking Water Drinking Water Filter

This Is Our Final Chart Table Of The Ammonia Nitrite Nitrate Ph And Temperature Levels Through Out The Course Of This Aquaponics Temperatures Fire And Ice

Microspheres For Use In Sea Water Reference Table Of Seawater Density By Temperature And Salinity Density Oceanography Markers

Buy Apera Instruments Ai311 Ph60 Premium Pocket Ph Tester Kit In 2022 Kit Tester Water Solutions

0 Begenme 0 Yorum Instagram Da Bimaks Water Chemicals Bimakskimya Http Www Bimakskimya Com Tr En Solutions Products Oil And Corrosion Drill Solutions

Pin On Fruit Vegetables Small Space Vertical Garden

How Does Temperature Affect Ph Westlab

How Does Temperature Affect Ph Westlab

Pin On You Learn Something New Every Day

What Causes A High Ph In A Swimming Pool
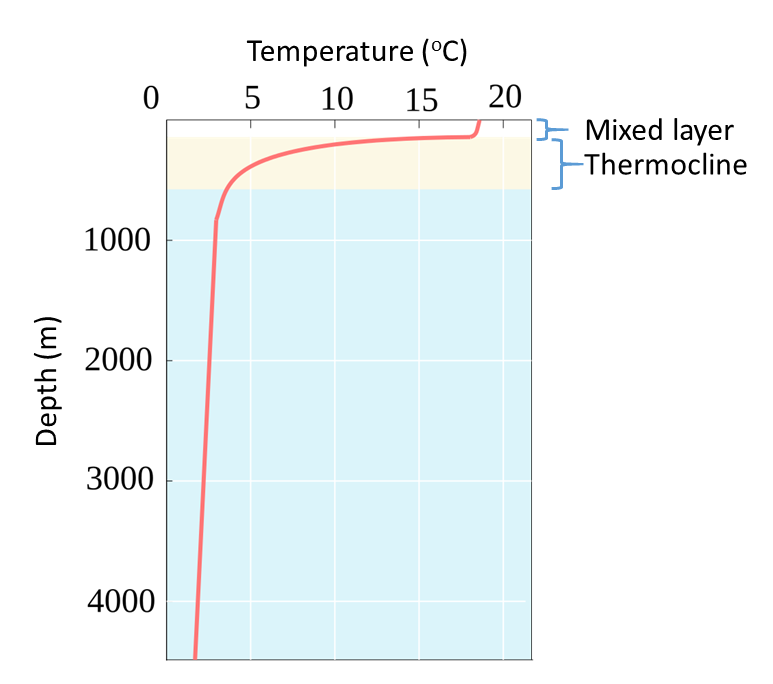
6 2 Temperature Introduction To Oceanography

Bluelab Monguacon Guardian Monitor Connect For Ph Temperature And Conductivity Measures Easy Calibration And Data Logging Connect Stick Not Included Gener In 2022 Data Temperatures Monitor

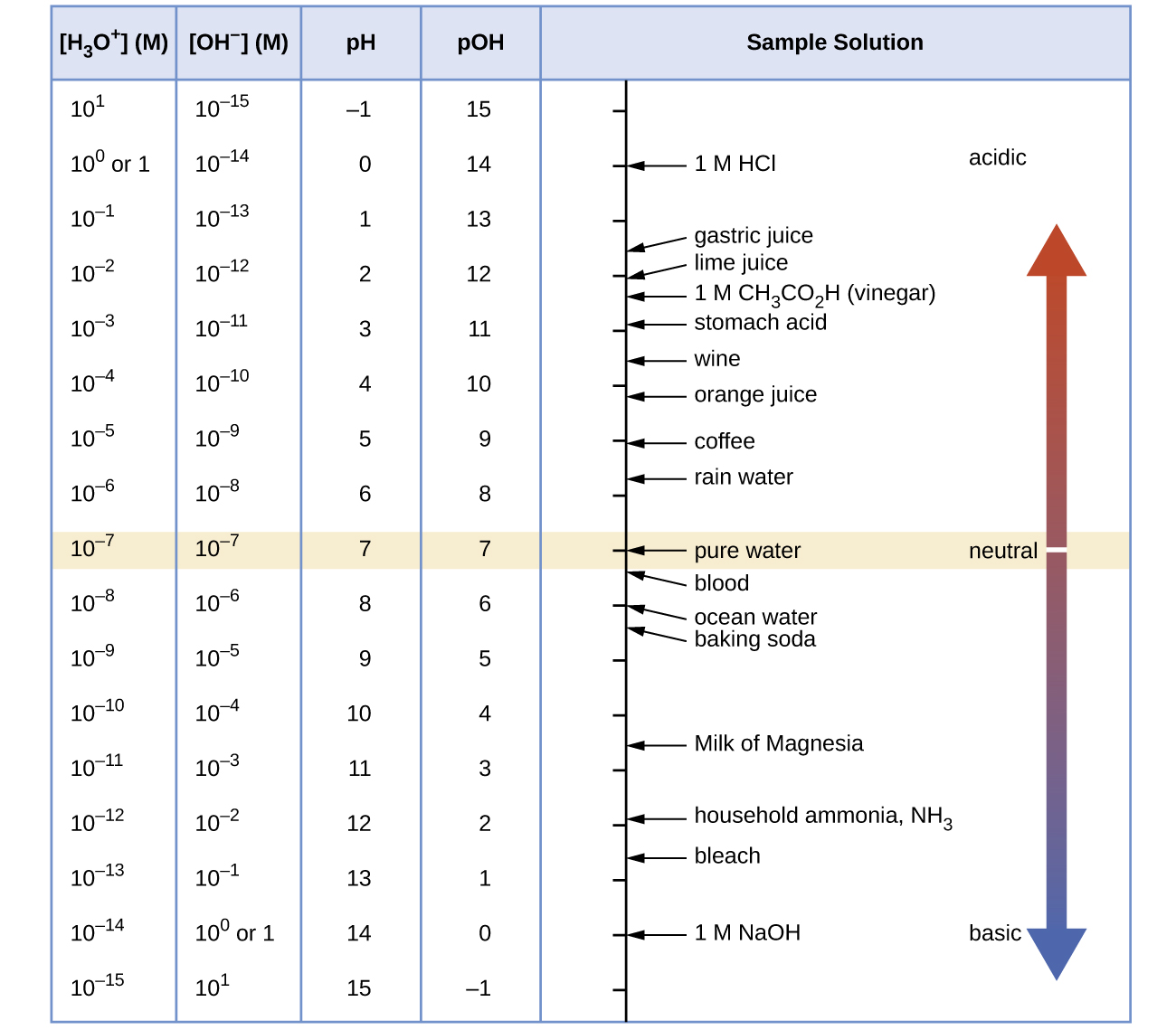


Comments
Post a Comment